Vapour Pressure of Liquid Solutions
Vapour Pressure of Liquid Solutions: Overview
This topic covers concepts such as Vapour Pressure, Variation of Vapour Pressure with Temperature, and Clausius-Clapeyron's Equation.
Important Questions on Vapour Pressure of Liquid Solutions
Select the correct Clausius-Clapeyron's Equation from the given options:
Write the Clausius-Clapeyron's Equation.
The plot of total vapour pressure as a function of mole fraction of the components of an ideal solution formed by mixing liquids and is
What are the factors which affect vapour pressure?
Explain the effect of temperature on the vapour pressure of a liquid.
What is a vapour pressure of liquid?
The vapour pressure of water at is of .
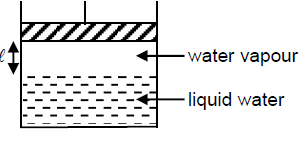
Which of the following will ensure that pressure of vapour above liquid will not change?
Vapour pressure of a liquid increases with
A person living at high altitude region observed that cooking food without using pressure cooker takes more time. The reason for this observation is that
Which of the following statements is correct?
At a constant temperature, which of the following aqueous solutions will have the maximum vapour pressure?
Statement - : Only temperature can change the vapour pressure of a pure liquid.
Statement - : Equilibrium constant does not change unless temperature is changed.
The enthalpy of vapourisation of
At pressure the boiling point of water is . What is the boiling point of water when pressure equal to in Kelvin in nearest possible integers?
Given:
A liquid is kept in a closed vessel. If a glass plate of negligible mass with small hole is kept on top of the liquid surface, then vapour pressure of the liquid in the vessel is:
Which one of the statements given below-concerning properties of solutions, describes a colligative effect?
The vapour pressure of a liquid in a closed container depends upon
On a humid day in summer, the mole fraction of gaseous a (water vapour) in the air at can be as high as 0.0287. Assuming a total pressure of 0.977 atm. What is the partial pressure of dry air?
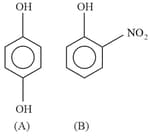
Out of the compounds below, the vapour pressure of (B) at a particular temperature is:
The aqueous solution that has the lowest vapour pressure at a given temperature is:
A substance will be deliquescent if its vapour pressure :
